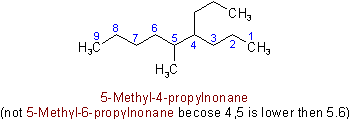
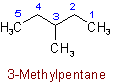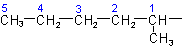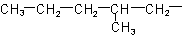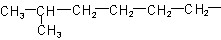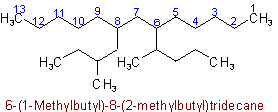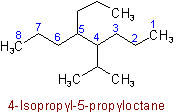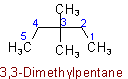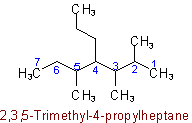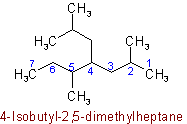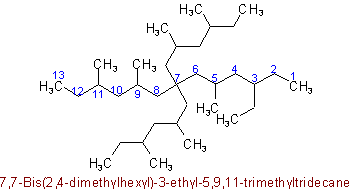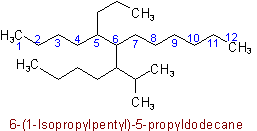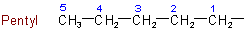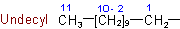R-4.2.1
R-4.2.2
R-4.2.3
Sunday, July 18, 2010
Basic numerical terms
Table 11 Basic numerical terms (multiplying affixes)
Acyclic Hydrocarbons
Rule A-2. Saturated Branched-chain Compounds and Univalent Radicals 
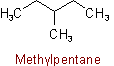
2.1 - A saturated branched acyclic hydrocarbon is named by prefixing the designations of the side chains to the name of the longest chain present in the formula.Example to Rule A-2.1The following names are retained for unsubstituted hydrocarbons only:
| Isobutane | 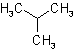 |
| Isopentane | 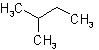 |
| Neopentane | 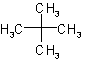 |
| Isohexane | 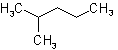 |
Examples to Rule A-2.22.25 - Univalent branched radicals derived from alkanes are named by prefixing the designation of the side chains to the name of the unbranched alkyl radical possessing the longest possible chain starting from the carbon atom with the free valence, the said atom being numbered as 1.
Examples to Rule A-2.25The following names may be used for the unsubstituted radicals only:
1-Methylpentyl 2-Methylpentyl 5-Methylhexyl
| Isopropyl | 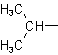 |
| Isobutyl | 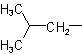 |
| sec-Butyl | 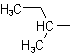 |
| tert-Butyl | 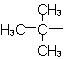 |
| Isopentyl |  |
| Neopentyl | 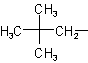 |
| tert-Pentyl | 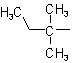 |
| Isohexyl | 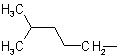 |
 .
.The alphabetical order is decided as follows:
(i) The names of simple radicals are first alphabetized and the multiplying prefixes are then inserted.
Examples to Rule A-2.3(i)(ii) The name of a complex radical is considered to begin with the first letter of its complete name.
ethyl is cited before methyl,
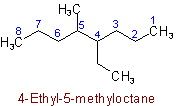
thus 4-Ethyl-3,3-dimethylheptane
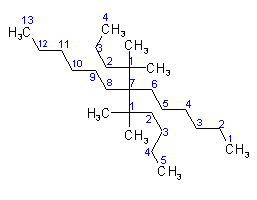
Examples to Rule A-2.3(ii)(iii) In cases where names of complex radicals are composed of identical words, priority for citation is given to that radical which contains the lowest locant at the first cited point of difference in the radical.
dimethylpentyl (as complete single substituent) is alphabetized under "d",
thus 7-(1,2-Dimethylpentyl)-5-ethyltridecane
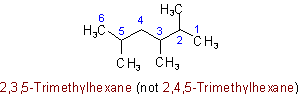
Examples to Rule A-2.3(iii)2.4 - If two or more side chains are in equivalent positions, the one to be assigned the lower number is that cited first in the name.
Examples: to Rule A-2.42.5 - The presence of identical unsubstituted radicals is indicated by the appropriate multiplying prefix di-, tri-, tetra-, penta-, hexa-, hepta-, octa-, nona-, deca-, undeca, etc.
Example to Rule A-2.5(a)The presence of identical radicals each substituted in the same way may be indicated by the appropriate multiplying prefix bis-, tris-, tetrakis-, pentakis-,etc. The complete expression denoting such a side chain may be enclosed in parentheses or the carbon atoms in side chains may be indicated by primed numbers.
Examples to Rule A-2.5(b)2.6 - If chains of equal length are competing for selection as main chain in a saturated branched acyclic hydrocarbon, then the choice goes in series to:(a) The chain which has the greatest number of side chains.
(a) Use of parentheses and unprimed numbers:
5,5-Bis(1,1-dimethylpropyl)-2-methyldecane
(b) Use of primes:
5,5-Bis-1',1'-dimethylpropyl-2-methyldecane
(a) Use of parentheses and unprimed numbers:
7-(1,1-Dimethylbutyl)-7-(1,1-dimethylpentyl)tridecane
(b) Use of primes:
7-1',1'-Dimethylbutyl-7-1",1"-dimethylpentyltridecane
Example to Rule A-2.6(a)(b) The chain whose side chains have the lowest-numbered locants.
Example to Rule A-2.6(b)(c) The chain having the greatest number of carbon atoms in the smaller side chains.
Example to Rule A-2.6(c)(d) The chain having the least branched side chains.
Example to Rule A-2.6(d)
hydrocarbon
Acyclic Hydrocarbons
Rule A-1. Saturated Unbranched-chain Compounds and Univalent Radicals 
1.1 - The first four saturated unbranched acyclic hydrocarbons are called methane, ethane, propane and butane. Names of the higher members of this series consist of a numerical term, followed by "-ane" with elision of terminal "a" from the numerical term. Examples of these names are shown in the table below. The generic name of saturated acyclic hydrocarbons (branched or unbranched) is "alkane".Examples of names:
(n = total number of carbon atoms)
1.2 - Univalent radicals derived from saturated unbranched acyclic hydrocarbons by removal of hydrogen from a terminal carbon atom are named by replacing the ending "-ane" of the name of the hydrocarbon by "-yl". The carbon atom with the free valence is numbered as 1. As a class, these radicals are called normal, or unbranched chain, alkyls.
Examples to Rule A-1.2
Subscribe to:
Comments (Atom)